Petrochemicals: Step-by-Step
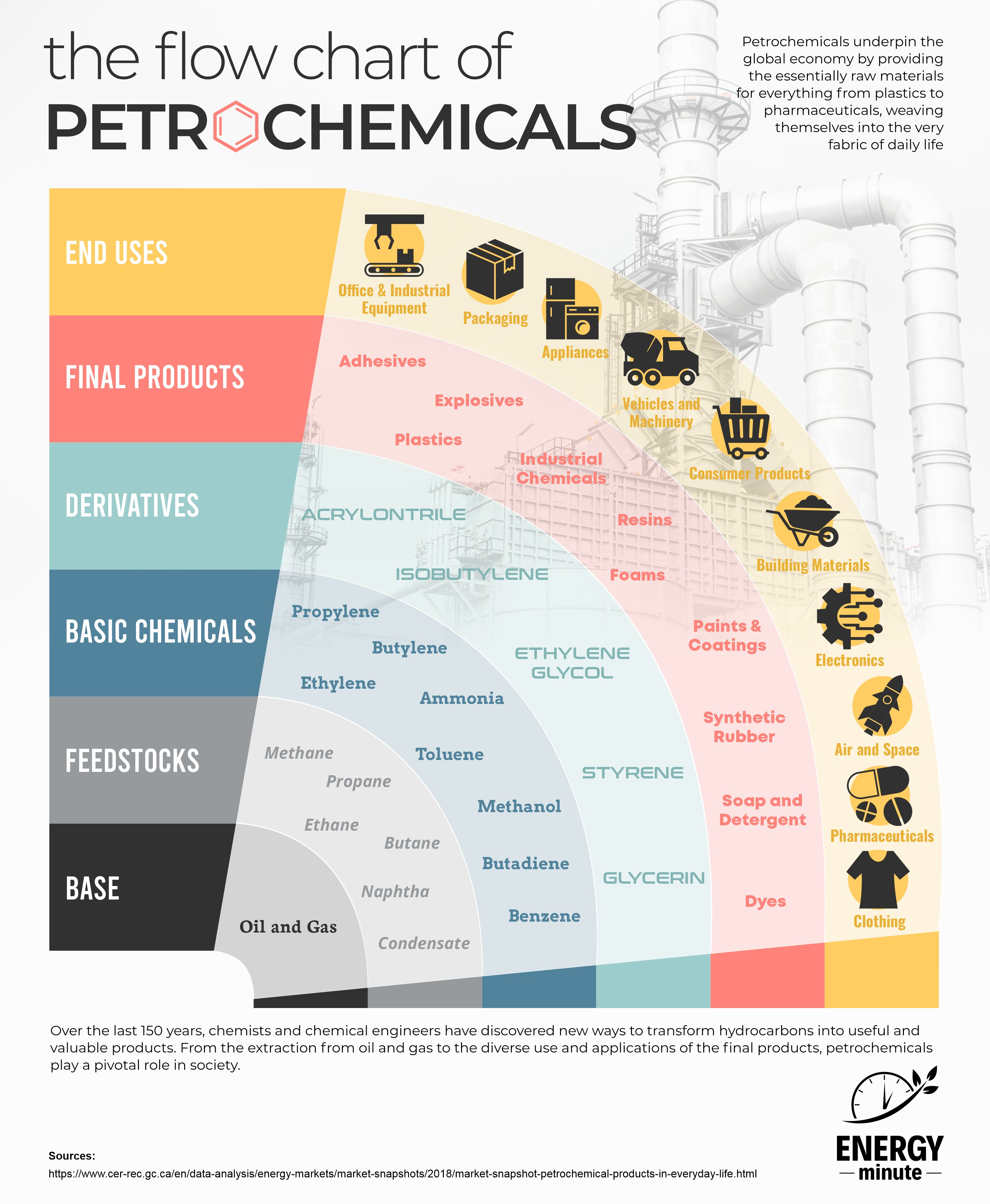
The process of turning oil and gas into petrochemicals involves several complex steps that transform hydrocarbons, which are the primary components of crude oil and natural gas, into a wide range of chemical compounds used in various industries. Petrochemicals are essential in the production of plastics, synthetic rubber, solvents, pharmaceuticals, detergents, and many other products.
Extraction and Refining: Petrochemicals are primarily derived from crude oil. Crude oil is extracted from underground reservoirs or offshore wells and transported to refineries. Some petrochemicals, particularly those involving methane or ethane, can be produced directly from natural gas.
Distillation: At the refinery, crude oil is first distilled in a distillation tower. The different hydrocarbon fractions in the crude oil are separated based on their boiling points. This process yields various products, including gasoline, diesel, jet fuel, and various petroleum gases.
Cracking: Petrochemical feedstocks are typically derived from the heavier fractions of crude oil or natural gas. To create these feedstocks, a process called cracking is employed. Cracking breaks down long-chain hydrocarbons into shorter-chain hydrocarbons. There are two main methods of cracking:
- Thermal Cracking: This process uses heat to break down hydrocarbons.
- Catalytic Cracking: In this method, a catalyst is used to accelerate the cracking reaction, allowing for more precise control over the products.
Reforming: In the reforming process, specific hydrocarbons are subjected to controlled temperature and pressure conditions to rearrange their molecular structure. This produces aromatic hydrocarbons, such as benzene, toluene, and xylene, which are crucial petrochemical building blocks.
Polymerization: Polymerization is the process of combining small, simple hydrocarbon molecules, typically alkenes, into larger molecules called polymers. Polymers are the basis for plastics and synthetic materials. Common examples include polyethylene, polypropylene, and polyvinyl chloride (PVC).
Oxidation and other chemical reactions: Petrochemicals can undergo various chemical reactions to produce a wide range of compounds. Oxidation processes, for example, are used to convert hydrocarbons into alcohols or organic acids. These intermediates can be further processed to create a variety of products.
Fractionation: After various chemical reactions, the mixture of products is often separated into different fractions through fractionation towers. This helps isolate specific chemicals for further processing or sale.
Purification: The final petrochemical products may require further purification to meet quality and industry standards. Various separation and purification methods, such as distillation, filtration, and solvent extraction, are used to achieve this.
Storage and distribution: The purified petrochemical products are stored in tanks or transported via pipelines, trucks, or ships to end-users and manufacturers who incorporate them into a wide range of consumer and industrial products.
The specific processes and reactions involved can vary widely depending on the desired petrochemical products. Petrochemical plants are highly complex and can produce a vast array of chemicals and materials, making them a critical part of the global manufacturing supply chain.




